Ronapreve / Ronapreve Uk Approves First Of Its Kind Antibody Cocktail To Treat Covid 19
Ronapreve is a combination of two antibodies which together prevent the virus from attaching to human cells and causing infection and also prevent further spread of infection in the body once a person has been infected. Clinical trial experience of use is limited to individuals aged.

Japan Approves Roche S Ronapreve For Covid 19 Treatment
BASEL dpa-AFX - Der Pharmakonzern Roche hat mit seinem Partner Regeneron in einer Phase-IIIII-Studie zum Medikament Ronapreve die gesteckten.

Ronapreve. Ronapreve is used to treat patients with confirmed acute covid-19 infection. Ronapreve 120 mgmL solution for infusion or injection multidose vials. Ronapreve casirivimab and imdevimab is being jointly developed by Roche and Regeneron.
Japan approves Roche-Regenerons Ronapreve for Covid-19 treatment. Antikörper-Cocktail von Roche EMA prüft Corona-Medikament Ronapreve. The two potent virus-neutralising antibodies are believed to bind non-competitively to the.
The applicant is Roche Registration GmbH. Ronapreve COVID-19 drug purchased by Australia for use in hospitals By political reporter Matthew Doran Posted 9h ago 9 hours ago Sat 16 Oct 2021 at 1136pm. Ronapreve is used to prevent acute covid-19 infection.
If necessary bags of diluted solution. About Ronapreve Ronapreve was designed specifically by Regeneron scientists to block the infectivity of SARS-CoV-2 the virus that causes COVID-19. Before diluting it allow the concentrated solution to come up to room temperature.
The two potent virus-neutralising antibodies are believed to bind non-competitively to the critical receptor binding domain of the viruss. Javid said he. The UKs most vulnerable hospital patients who are unable to build up an antibody response to Covid will be offered new drug Ronapreve from.
Patients were randomized 111 to receive a. Ronapreve co-developed by Regeneron Pharmaceuticals Inc. About Ronapreve casirivimab and imdevimab Ronapreve is a combination of two monoclonal antibodies also known as REGN10933 and REGN10987 respectively and was designed to block infectivity of SARS-CoV-2 the virus that causes COVID-19.
Studies have taught us that when used as a treatment and provided that it is given as quickly as possible after first symptoms of illness the treatment can reduce the. Ronapreve has undergone a number of clinical trials around the world. EMA has started evaluating an application for marketing authorisation for the monoclonal antibody combination Ronapreve casirivimab imdevimab.
Ronapreve ist derzeit nicht für Patienten zugelassen die aufgrund einer COVID-19-Infektion im Krankenhaus behandelt werden. Großbritannien hat Ronapreve zugelassen. The antibody cocktail is authorised for emergency use in several regions including the EU and the US.
Known as Ronapreve it is the first artificial antibody drug of its type to be approved in the UK which was hailed by the health secretary Sajid Javid as fantastic news. Before use store unopened Ronapreve concentrated solution in a refrigerator until the day it is needed. Ronapreve which was used to treat Donald Trump will be saving lives as early as next week says Sajid Javid Last modified on Sat 18 Sep 2021 0418 EDT A drug given to the former US.
And Roche Registration GmbH is intended for the treatment of COVID-19 in adults and adolescents from 12 years of age who do not require. 21 Jul 2021 Last Updated July 21st 2021 0920 The approval is based on a Phase I trial of the antibody combination in Japanese participants and a global Phase III trial. Equitable allocation is a priority On 20 August 2021 Ronapreve received conditional marketing authorisation for the prevention and treatment of covid-19 in the UK1 Ronapreve REGEN-COV in the US comprises two monoclonal antibodies casirivimab and imdevimab that target the SARS-CoV-2 spike protein to reduce the risk and severity of covid-19 in selected patients2345 Although Ronapreve.
Anfang dieses Jahres hat der Ausschuss für Humanarzneimittel der Europäischen Arzneimittel-Agentur eine wissenschaftliche Stellungnahme abgegeben in der er die Verwendung von Ronapreve als Behandlungsoption für nicht hospitalisierte Patienten mit bestätigter. Der Hauptsitz des Schweizer Pharmaunternehmens ist in Basel. According to Regeneron by July 2021 16000 patients in both hospitalised and.
It is a combination of two monoclonal antibodies casirivimab and imdevimab also known as REGN10933 and REGN10987 and was designed to block infectivity of SARS-CoV-2 the virus that causes COVID-19. Ronapreve is the first of its kind for the treatment of Covid-19 and after a meticulous assessment of the data by our expert scientists and clinicians we are satisfied that this treatment is. Dpa 20082021 1730 Uhr.
They evaluated thousands of fully-human antibodies produced by the companys proprietary VelocImmune mice which have been genetically-modified to have a human immune system as well as antibodies identified from humans who have. DJ Roches Covid-19-Medikament Ronapreve erhält Zulassung in Japan Von Giulia Petroni BASEL Dow Jones--Der Schweizer Pharmakonzern Roche hat in Japan die Zulassung für sein Medikament Ronapreve. Ronapreve has been approved for use in different patient populations in Japan and conditionally in the UK and is authorized for emergency use in the US India and Canada.
Ronapreve 120 mgml solution for infusion or injection single-use vial. Pack of two 20 mL clear Type I glass vials with butyl rubber stopper containing one vial of 111 mL solution of 1 332 mg of casirivimab and one vial of 111 mL solution of 1 332 mg of imdevimab. About the REGN-COV 2066 study The phase IIIII randomized double-blind placebo-controlled trial evaluated Ronapreve in hospitalized adult patients with COVID-19.
Once diluted Ronapreve should be used immediately. Ronapreve belongs to a class of drugs called monoclonal antibodies which mimic natural antibodies produced by the body to fight off infections. Ronapreve will be stored by the healthcare professionals at the hospital or clinic under the following conditions.

Ronapreve Uk Approves First Of Its Kind Antibody Cocktail To Treat Covid 19
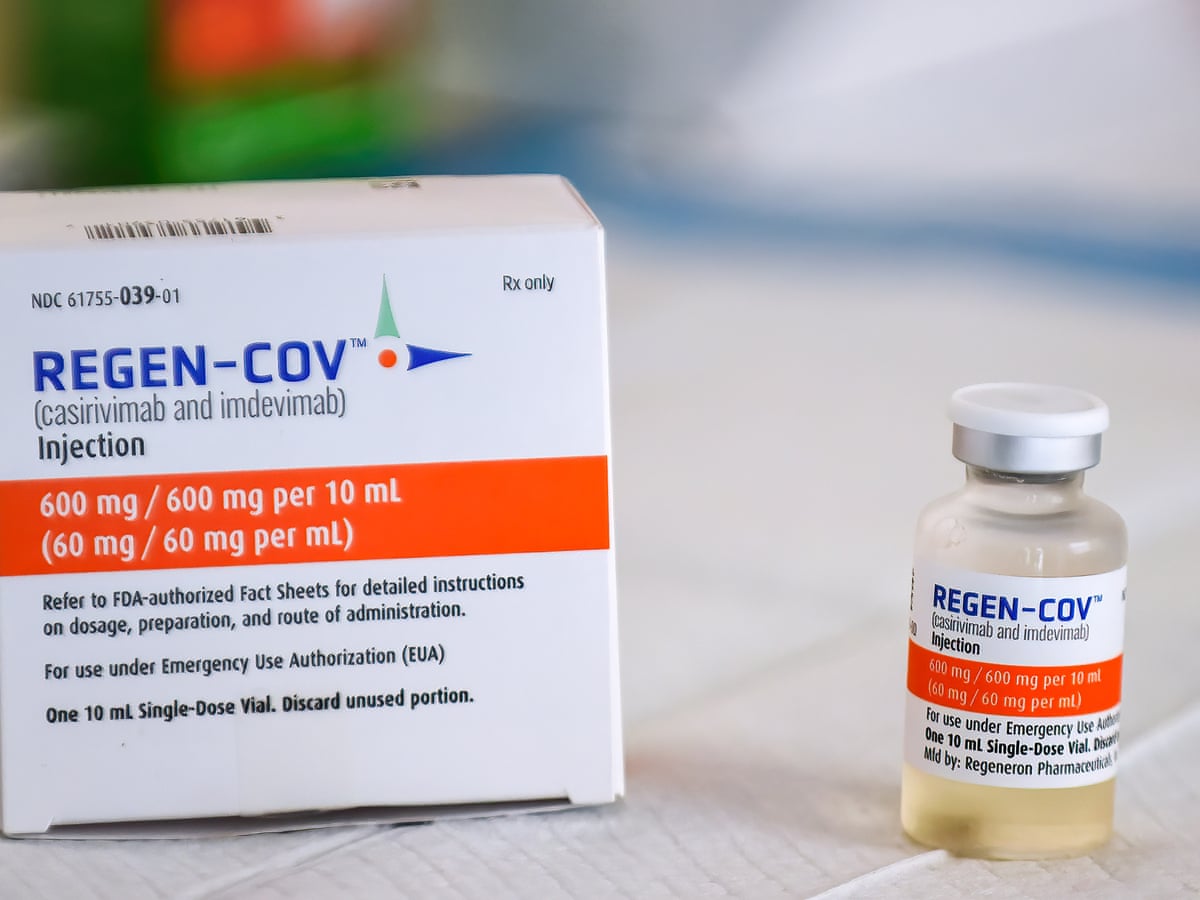
Uk Regulator Approves First Of Its Kind Covid Antibody Treatment Coronavirus The Guardian

Scientists Welcome Uk Approval Of Covid 19 Antibody Cocktail Research Professional News

Roche L Ue Debute La Procedure D Examen Du Medicament Ronapreve Contre Covid 19 Bilan
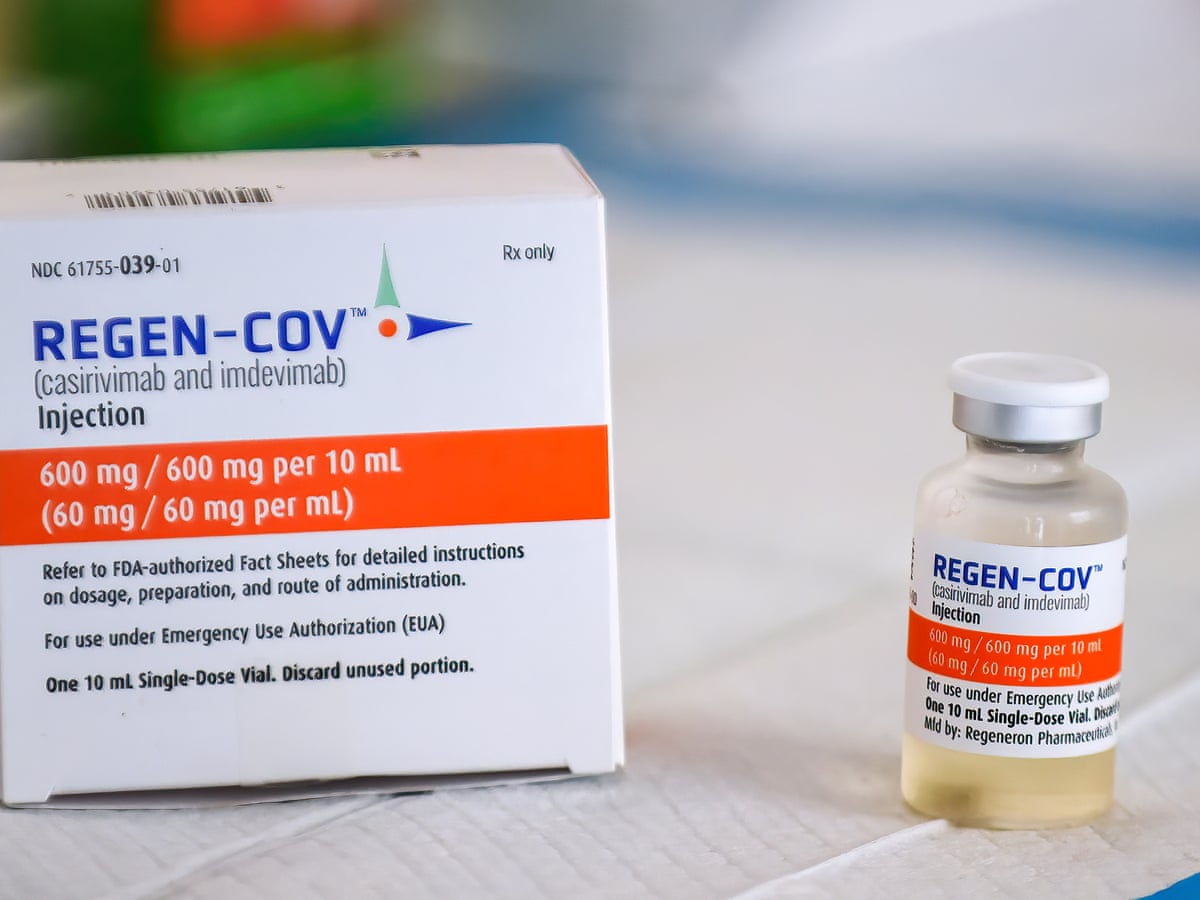
Covid Antibody Drug Ronapreve To Be Given To Vulnerable Nhs Patients Nhs The Guardian
Ronapreve New Covid 19 Treatment Has Just Been Authorised Here S Everything You Need To Know
Ronapreve Reduces Covid 19 Viral Load Within Seven Days

Chugai Files For Additional Indications For Ronapreve In Japan
What Are Ronapreve And Pf 07321332 The Two New Covid 19 Treatments Australia Has Gained Access To Abc News

Japan Backs Revolutionary Covid 19 Drug Ronapreve To Help Tame Crisis The Japan Times
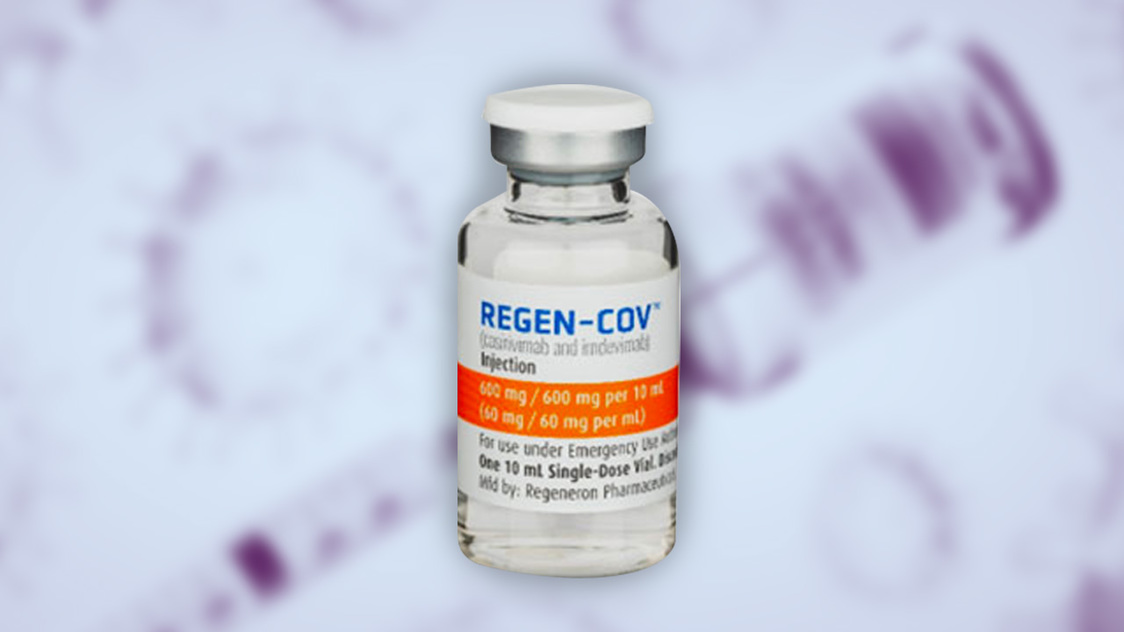
Philippines Grants 1st Emergency Use Treatment For Covid 19 To Ronapreve
What Are Ronapreve And Pf 07321332 The Two New Covid 19 Treatments Australia Has Gained Access To Abc News
Ronapreve New Covid 19 Treatment Has Just Been Authorised Here S Everything You Need To Know

Japan Approves Roche Regeneron S Ronapreve For Covid 19 Treatment
Eu Regulator Starts Evaluating Covid 19 Drug Ronapreve

Eu Regulator Starts Evaluating Covid 19 Drug Ronapreve




Post a Comment
Post a Comment